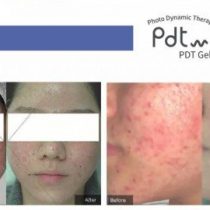
Mesotherapy has allowed countless patients to find relief from embarrassing cellulite. It is true that many people mistakenly believe it is a fat problem when in reality it’s a skin problem. The thighs, hips and buttocks are most commonly affected, and the problem is thought to affect most of the adult population, both men and women.
This skin condition may be particularly embarrassing due to the cottage cheese look of the problem. Some turn to creams or pills that promise to remove the small dimple features without investigating the method and end up disappointed. Before choosing any treatment, patients should consult a trained practitioner to determine if it is right for them.
Fortunately, a newer procedure is available to effectively eliminate cellulite Mesotherapy is non-invasive and does not require any incisions or anesthetic. Instead, injections consisting of vitamins and homeopathic and conventional medicines are made into the middle layer of skin (mesoderm) are made that is also effective in relieving wrinkles, excess fat and sagging skin.
VLine A solution lipolysis injection combines phosphatidylcholine & L-Carnitine together to maximize the result. It dissolves fat and removes cellulite, also it gives elasticity to the skin by generating collagen for lifting effect. There may be some degree of heaviness and general uncomfortableness linked with lipolysis injection, although no recovery time is necessary. The body works to change the body’s interior structures after the procedure to improve the outward appearance of the skin. Consult with a doctor or physician for more information about how the treatment is performed and what to expect after treatment.

It may take about at least 3 treatments before patients begin to notice results, as the treatment is non-invasive. This may vary among patients, however. Over a period of a few weeks to a few months, patients may begin to gradually notice results.
A majority of insurance companies will not insure mesotherapy, as it is normally categorized as a cosmetic treatment. Due to it not being a medical priority or necessity, treatment is not considered necessary. To learn more about what your insurance may cover, speak with your insurance carrier.
4,404 total views, 4 views today